14 min read
Behavior tests used with the 6-OHDA model of PD, and what they tell us.
By: MD Biosciences on Sep 30, 2013 10:28:00 PM
6-OHDA lesioned rats exhibit a number of motor behavioral and non-motor impairments which have been examined with a variety of tests, summarized below.
Outcomes on behavioral tests may depend on a number of factors. Certainly, the severity of impairments is influenced by lesion size. The threshold of lesion needed to detect changes can vary among tests, and some tests are not very sensitive for partial lesions [27]. Some tests appear to be more sensitive to lesions in one area than another [8]. Outcomes also can be influenced by sublocation within the injection site, and spread of toxin. For example, injections in the SNpc can spread into the ventral tegmental area. In mfb injections, nigrostriatal and mesolimbic pathways are both affected [22]. The striatum is considered by many to be made up of 6 subregions (dorsomedial, dorsocentral, dorsolateral, ventromedial, ventrocentral, ventrolateral), with outcomes differing based on the subregion targeted and the number of subregions injected [14].
The spontaneous behavioral changes in 6-OHDA unilaterally lesioned rats include reduced use of the contralateral paw, a preference to notice items, such as food, on the ipsilateral side, and a propensity to turn in the ipsilateral direction [9].
Rotation Test
Rotational behavior has been widely used to measure the motor consequences of unilateral 6-OHDA lesions in the rat. Following dopamine depletion, animals exhibit a postural bias toward the side of lesion. The rotation test takes advantage of this postural asymmetry and examine circling behavior induced by systemic injection of either apomorphine or amphetamine.
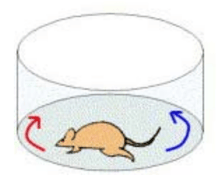
Note that this test is different from the other motor behavioral tests in that it requires administration of an external agent. Apomorphine (and L-DOPA) are dopamine receptor agonists, i.e. they are drugs that activate dopamine receptors in the absence of dopamine. In the rotation test, administering these agents causes the animal to rotate away from lesioned side (”contraversive rotation”). Differently, the administration of amphetamine, which presynaptically stimulates the release of endogenous dopamine, produces a turning toward the lesioned side (”ipsiversive rotation”). One way this has been conceived is that the rat turns away from the side where dopamine activity is greater. The parameter that is monitored is the number of rotations in a set time, typically measured using automated rotometer systems [reviewed in 9, 19].
The rotation test has been so frequently used because the behavior is directly observable and easy to measure, and the test is consistent, objectively, and gauges striatal dopamine receptor sensitivity. It is often used to screen for lesions, estimate lesion size, and assess the efficacy of neuroprotective and neuron replacement strategies. The test also has been used to evaluate potential new medications for their anti-dyskinetic potential. Rotational behavior is related to the degree of the lesion, but the correlation has not been shown to be linear, likely because a number of compensatory mechanisms come into play.
Amphetamine response (ipsiversive rotation):
A response to amphetamine can be detected with as little as 50% loss of dopaminergic neurons, which makes this test good for detecting partial lesions. (Although some reports suggest that a response to partial lesions, i.e. 50%, is not consistent). But, because, the intensity of rotation does not increase significantly with larger lesions, even up to 90%, amphetamine-induced rotation cannot be used to screen for large (~90%) lesions. The lack of graded response implies also that, if being used to assess neuroprotective or replacement therapy, amphetamineinduced rotation cannot detect improvements that are small: a sizable percent (>50%) of the nucleus would need to be restored before the test would register a change in rotational behavior.
Apomorphine response (contraversive rotation): Rotation to apomorphine is only consistently seen when >90% of dopaminergic neurons have been lost. This is the point at which denervation-induced dopamine receptor supersensitivity on the ipsilateral side occurs. Therefore, unlike amphetamine, the apomorphine response can be used to pre-screen for full (>90%) lesions.
It is well known that, in humans, long term treatment with L-DOPA produces dyskinetic movements, which limit the long term usefulness of this medication. Much research has been directed at understanding the cause of these dyskinesias, and at developing treatments that can reduce or prevent their development. Because administration of L-DOPA to 6-OHDA-lesioned rodents produces contraversive rotation, the rotation test has been used to assess potential new treatments, by screening for compounds that reverse the L-DOPA-induced rotational behavior. Results from these types of experiments are not always consistent [16]. One possible reason is rotational behavior was assessed at different times, times when drugs – either L-DOPA, the potential anti-dyskinetic agent, or both – might not have been interacting optimally. In addition, noting that drug-induced rotation reflects the net effect of dopamine depletion, receptor supersensitivity, and receptor loss, it is possible that tests other than drug-induced rotational behavior, e.g. analysis of abnormal involuntary movements (AIMs), or cylinder test, are better suited for developing new therapies to treat dyskinesias.
Some epidemiological studies have suggested that nicotine may be neuroprotective for dopaminergic neurons. When used in the rotation test, nicotine has been shown to decrease the number of apomorphine-induced rotations, but this may only happen when sufficient drug is delivered to cause the nicotinic acetylcholine receptors to go into a desensitized state [12].
Cylinder test
The cylinder test (sometimes referred to as the rearing test, paw placement or the limb use asymmetry test) detects forelimb impairments, specifically, asymmetry in limb use for weight bearing movements. The test is easy and quick (~5 min) to carry out, and requires no specialized training to make an accurate assessment. Because the primary outcome measurement involves comparing use of the affected to unaffected side, each animal serves as its own control. This test has been shown to be sensitive to the degree of dopamine loss [19], and has been used to evaluate various types of transplants and viral vectors [11]. In this test, the rat is placed without habituation in a clear cylinder ~ 20 cm in diameter and ~30 cm high for 3–10 min.
As the rat explores the cylindrical shape with the forelimbs it is videotaped from the side or below for the experimental time, and paw use is scored as the number of wall contacts made by the impaired forelimb, as a percentage of total forelimb contacts. Other factors sometimes measured include weight shifting during rearing, percent use of each forelimb for landing, or amount of time spent with paws in contact with the walls. 6-OHDA lesioned animals prefer to use their non-impaired (ipsilateral) forelimb for vertical exploration of the cylinder. A threshold lesion size may be required in order to observe a performance change on this task.
Staircase Test
The staircase test (also referred to as the paw reaching test) assesses skilled reaching and grasping [20]. It involves a simple apparatus, consisting of a small box, with two interior narrow staircases, one along each of the long edges, and a central, lengthwise, rat-width plinth between them, raised above the level of the highest stair. The rat is placed on the plinth, from which it has the opportunity to reach down onto the steps on either side for food pellets placed on each step. Because the apparatus is narrow, the rat is restricted from turning, and thus can only retrieve pellets from the right staircase with the right paw, and vice versa.
Reach and grasp coordination of the forelimbs is measured by counting the number of pellets retrieved and the number displaced on each side, with each rat serving as its own control. Unilateral dopamine lesions impair rats’ abilities to reach and retrieve pellets with the contralateral forelimb. Scores for the contralateral side have been found to correlate non-linearly with dopaminergic cell loss, although impairments on this test may not be apparent until ~50% loss of nigral dopamine neurons or 60-80% loss of striatal dopamine [15].
Adjusting steps test
The adjusting steps test (also referred to as the stepping test) is used to assess postural stability, gait problems, and limb bradykinesia. It is a reliable indicator of unilateral dopamine loss [19, 21]. To perform the test, the investigator holds animals so that both hind limbs and one forepaw are raised just off the surface of a wide table. The animal is moved laterally across the surface of the table (over ~80-90 cm for ~10 sec) in such a way that it must bear weight on its remaining forepaw. The number of adjusting steps made by the weight-bearing forepaw is counted, with each adjusting step logged as either a forehand step (movement of the paw towards the torso to compensate for a movement of the body away from the side of weight-bearing forepaw) or a backhand step (movement of the paw away from the torso to compensate for a movement of the body toward the side of the weight-bearing forepaw.) The test can also be performed with the weight-bearing forepaw placed on a treadmill set at a constant speed. The average number of adjusting steps over 3-5 trials is commonly used for analysis. Animals with nigrostriatal lesions typically drag the weight-bearing forelimb across the table instead of making adjusting steps.
This test is also used to investigate additional parameters relevant to PD, such as movement initiation, stepping time, and step length. Movement initiation is measured as the time until the rat initiates movement. Stepping time is measured as the time it takes the rat to “walk” a given distance after initiation. Step length is calculated by dividing the distance by the number of steps [21].
Results on this test are influenced by lesion location and severity. When lesions are made in the medial forebrain bundle (mfb), reductions in the ability to make adjusting steps may not be observed until striatal dopamine is reduced by ~80%, but impairments may be observable as early as 3 days post lesion [5]. For striatal lesions (specifically, for discrete lesions made in the dorsolateral, ventrolateral or ventrocentral striatum), the reduction in the number of adjusting steps by the contralateral paw is not as severe as after mfb lesions [5, 14]. Some investigators see deficits in this task with striatal lesions of 50-70%, while others do not detect any change in performance of animals with <80% lesion. The threshold of lesion needed to detect deficits in this test is lower than for the paw reaching test [14].
Corridor test
The corridor test is based on the fact that rodents with unilateral nigrostriatal lesions fail to orient to stimuli (e.g. tactile, visual, olfactory) presented on the side of the body contralateral to the lesion, for example, they neglect food placed on the contralateral side. In this task, sugar pellets are placed on both sides of the rat’s body, and the number of pellets retried from each side is assessed. Rats with lesions of the nigrostriatal system preferentially retrieve pellets from the ipsilateral side of the body. This test has been used to assess the severity of lesions and the speed at which they develop. For example, rats with an mfb lesion were impaired in this task after three weeks, whereas striatal-lesioned rats only showed impairments after about 8 weeks, likely reflecting the slower degeneration in the striatal model [16].
Test of non-motor function
The 6-OHDA model of PD has also been used to model non-motor symptoms of PD, including cognitive symptoms such as procedural memory, working memory and executive function; and affective symptoms such as depression and anxiety. However, there is discussion about how well a dopamine-based model like the 6-OHDA model can replicate non-motor PD symptoms because non-motor symptoms probably involve non-dopamine transmitter systems, e.g. serotonin, norepinephrine, and acetylcholine, and different pathophysiology, and because some of the non-motor symptoms of PD do not respond to dopamine-enhancing treatments. Nevertheless, evidence suggests that the 6-OHDA model can accurately replicate some of the non-motor symptoms of PD.
The various tests that have been used with the 6-OHDA model are summarized below. It should be kept in mind that many, if not all, of these tests can be complicated by behavioral deficits and disturbances in olfactory function produced by the model. To evaluate and take into account these confounds, many investigators employ a locomotor activity test, such as the open field test, or an olfactory discrimination test.
Morris water maze
The Morris water maze has been widely used to assess spatial and procedural learning and memory in rodents. The apparatus is well described elsewhere, but basically consists of black, circular pool ~150 cm in diameter and ~50 cm in height, into which is placed a submerged platform large enough for the rodent to stand on. The pool is filled with clouded water so that the platform is not visible and so that the subject cannot touch the bottom. The task consists of placing the subject in the pool and recording the amount of time needed to swim to the platform. Rodents typically learn the location of the platform over the course of a few testing sessions, as indicated by a decrease in the amount of time required to swim to the platform. Different protocols have been developed to investigate spatial learning and memory, and procedural learning and memory: spatial testing incorporates visual cues on the walls outside the pool, and procedural memory testing makes use of a visual cue above the platform to indicate the platform’s location.
Outcomes in this task are likely to be influenced by size of the lesion, and are sensitive to motor impairments. Lesioned animals have been found both to spend more time than controls to find the platform [26, and reviewed in 9], and the same amount of time as controls [3].
In the case where lesioned animals underperformed controls, lesioned animals were able to learn the task as their latency to find the platform improved over the training days. Forced swimming test The forced swim test, or a modified version of it, is the most widely used test for depression. It is simple and rapid, and has been used to screen compounds for antidepressant activity. In the test, the rodent is placed in a vertical glass cylinder (~20-30 cm diameter, ~40 cm height) filled with water (~25 degrees C; ~20 cm deep) so that the rat cannot reach the bottom with the hind paws. Two sessions, one training and one testing, are used. The testing session is videotaped and behavioral responses - climbing, swimming and floating (immobility) - are each quantified. Short latency to floating and long floating duration are considered measurements of depressionlike behavior (‘behavioral despair’).
In 6-OHDA lesioned rats, shorter latency to floating and longer floating duration have been observed [3, 23, 26], although sometimes measures of immobility do not reach statistical significance [29].
Social recognition test
The social recognition test assesses short-term social memory. The test consists of two sessions of presenting a juvenile to an adult rat and recording the time spent by the adult in investigating the juvenile. When the delay between the two sessions is less than ~40 minutes, the amount of time investigating the juvenile is usually less during the second presentation, demonstrating social recognition. 6-OHDA lesioned rats have been noted to spend as much time investigating the juvenile rat during the second session as during first, reflecting an impairment in recognition (i.e. memory) [26].
Sucrose preference test
Sucrose preference is often used as measure of anhedonia, and thus of depression-like behavior, in rodents. The test can be done in a number of ways, but the goal is to determine whether, when given a choice, rats will drink more sucrose containing water (1-2% sucrose) than normal water.
Results in this test are not consistent. For example, bilateral 6-OHDA injection in the striatum did not affect the amount of sucrose solution drunk by rats [3], perhaps because both control and lesioned rats should a clear preference for sweet water. On the other hand, although both control and lesioned animals preferred the sucrose solution, sham lesioned animals consumed more sucrose than 6-OHDA lesioned animals [26].
Elevated plus maze
The elevated plus maze is the most common test of anxiety-related behavior in rodents. The apparatus is well described in many places, but basically it consists of a set of 2 elevated walkways that intersect to form a plus shape. Two of the walkways are open, and two are enclosed. Given rodents’ dislike of open spaces, the relative time spent in the open compared to the closed arms is considered a measure of anxiety. For 6-OHDA lesioned rats, both increases and decreases in time in the open arms have been observed [3, 26].
Tests of executive function
Tests of executive function are less widely used with the 6-OHDA model than are the tests described above. In PD, the dysexecutive syndrome is characterized by deficits in attention, reversal learning, and working memory, as well as in rule learning and decision making.
Several aspects of this dysexecutive syndrome have been investigated in 6-OHDA lesioned rats [reviewed in 16]. In brief:
- Attentional deficits have been demonstrated in rats with bilateral striatal lesions, using the fivechoice serial reaction time task and other related tasks.
- Reversal learning has been shown to be impaired in rats with bilateral striatal lesions, using different types of mazes (e.g. water T-maze), operant boxes and digging bowls.
- Working memory has been assessed using the delayed alternation task, using the T-maze or operant boxes. Decreases in task accuracy - the most common measure of task performance - were observed in rats with striatal 6-OHDA lesions.
MD Biosciences offers the 6-OHDA model in rats and has validated some of the parameters covered in this review and is continuing development to include further parameters and will work with sponsors to include other additional tests/parameters upon request.
Note: This review has provided an overview of the 6-OHDA model of PD, with an emphasis on results obtained from rodent, especially rat. It should not be considered as comprehensive or definitive.
References
- Blandini, F., Levandis, G., Bazzini, E., Nappi, G. & Armentero, M.-T. Time-course of nigrostriatal damage, basal ganglia metabolic changes and behavioural alterations following intrastriatal injection of 6-hydroxydopamine in the rat: new clues from an old model. The European journal of neuroscience 25, 397–405 (2006)
- Blandini, F., Armentero, M.-T. & Martignoni, E. The 6-hydroxydopamine model: news from the past. Parkinsonism & related disorders 14 Suppl 2, S124–9 (2007).
- Branchi, I. et al. Nonmotor symptoms in Parkinson’s disease: investigating early-phase onset of behavioral dysfunction in the 6-hydroxydopamine-lesioned rat model.Journal of neuroscience research 86, 2050–61 (2008).
- Bové, J. & Perier, C. Neurotoxin-based models of Parkinson’s disease. Neuroscience 211, 51–76 (2012).
- Chang, J., Wachtel, S., Young, D. & Kang, U. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson’s disease: studies on medial forebrain bundle and striatal lesions. (1999). at
- Cicchetti, F., Brownell, A.L., Williams, K., Chen, Y.I., Livni, E., Isacson, O. Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. European Journal of Neuroscience 15, 991-998 (2002).
- Costa G; Abin-Carriquiry JA; Dajas F Nicotine prevents striatal dopamine loss produced by 6-hydroxydopamine lesión in the substantia nigra. Brain research. 888(2). 336-342. (2001).
- Deumens, R., Blokland, A. & Prickaerts, J. Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Experimental neurology175, 303–17 (2002).
- Dunnett, S. & Lelos, M. Behavioral analysis of motor and non-motor symptoms in rodent models of Parkinson’s disease. Progress in brain research 184, 35–51 (2009).
- Duty, S. & Jenner, P. Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. British journal of pharmacology 164, 1357–91 (2011).
- Fleming, S., Schallert, T. & Ciucci, M. Cranial and related sensorimotor impairments in rodent models of Parkinson’s disease. Behavioural brain research 231, 317–22 (2012).
- Han, F. & Wang, H. Effects of desensitized nicotinic receptors on rotational behavior in a 6-hydroxydopamine model of Parkinson’s disease. Neuroscience letters 415, 200–4 (2007).
- Jeon, B., Jackson-Lewis, V. & Burke, R. 6-Hydroxydopamine lesion of the rat substantia nigra: time course and morphology of cell death. Neurodegeneration : a journal for neurodegenerative disorders, neuroprotection, and neuroregeneration 4, 131–7 (1995).
- Kirik, D., Rosenblad, C. & Björklund, A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Experimental neurology 152, 259–77 (1998).
- Lee, C., Sauer, H. & Bjorklund, A. Dopaminergic neuronal degeneration and motor impairments following axon terminal lesion by instrastriatal 6-hydroxydopamine in the rat. Neuroscience 72, 641–53 (1996).
- Lindgren, H. & Dunnett, S. Cognitive dysfunction and depression in Parkinson’s disease: what can be learned from rodent models? The European journal of neuroscience 35, 1894–907 (2012).
- Lundblad, M., Andersson, M., Winkler, C., Kirik, D., Wierup, N. and Cenci, M. A., Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. EJN, 15: 120–132. doi: 10.1046/j.0953-816- x.2001.01843.x (2002).
- McDowell, K. & Chesselet, M.-F. Animal models of the non-motor features of Parkinson’s disease. Neurobiology of disease 46, 597–606 (2012).
- Meredith, G. & Kang, U. Behavioral models of Parkinson’s disease in rodents: a new look at an old problem. Movement disorders : official journal of the Movement Disorder Society 21, 1595–606 (2006).
- Montoya, C., Campbell-Hope, L., Pemberton, K. & Dunnett, S. The ‘staircase test’: a measure of independent forelimb reaching and grasping abilities in rats. Journal of neuroscience methods 36, 219–28 (1991)
- Olsson, M., Nikkhah, G. & Bentlage…, C. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. (1995).at
- Perese, D., Ulman, J., Viola, J., Ewing, S. & Bankiewicz, K. A 6-hydroxydopamine-induced selective parkinsonian rat model.Brain research 494, 285–93 (1989).
- Santiago, R. et al. Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson’s disease are predominantly associated with serotonin and dopamine. Progress in neuro-psychopharmacology & biological psychiatry 34, 1104–14 (2010).
- Sarre, S. et al. In vivo characterization of somatodendritic dopamine release in the substantia nigra of 6-hydroxydopamine-lesioned rats. Journal of neurochemistry 90, 29–39 (2004).
- Schober, A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell and tissue research 318, 215–24 (2004).
- Tadaiesky, M. et al. Emotional, cognitive and neurochemical alterations in a premotor stage model of Parkinson’s disease.Neuroscience 156, 830–40 (2
- Truong, L., Allbutt, H., Kassiou, M. & Henderson, J. Developing a preclinical model of Parkinson’s disease: a study of behaviour in rats with graded 6-OHDA lesions.Behavioural brain research 169, 1–9 (2006).
- Visanji, N., O’Neill, M. & Duty, S. Nicotine, but neither the alpha4beta2 ligand RJR2403 nor an alpha7 nAChR subtype selective agonist, protects against a partial 6-hydroxydopamine lesion of the rat median forebrain bundle. Neuropharmacology 51, 506–16 (2006).
- Zhang, X., Egeland, M. & Svenningsson, P. Antidepressant-like properties of sarizotan in experimental Parkinsonism.Psychopharmacology 218, 621–34 (2011).
- Zuch, C. et al. Time course of degenerative alterations in nigral dopaminergic neurons following a 6-hydroxydopamine lesion. The Journal of comparative neurology 427, 440–54 (2000).


