At MD Biosciences we support IND-enabling studies and assist in several ways. Our dedicated team of scientists helps plan the studies with appropriate endpoints, biomarkers, and methodologies that align with regulatory requirements. We perform preclinical studies in rodents and pigs, and can include components like pharmacology, toxicology, and pharmacokinetics. At MD Biosciences we generate data and results for FDA submission and have quality control and quality assurance processes in place to ensure that each step of the study meets the requirements.
PK/PD
MD Biosciences provides PK services for rodents and pigs, and designs studies that optimize sample collection and analytics. Below is a list of our PK services:
- Standard PK
- Dose linearity PK
- CNS penetration
- Tissue distribution
- Formulation screening
- Bioanalysis services: sample analysis by LC-MS/MS of biofluids or tissues
Toxicology
Toxicology studies are crucial in assessing drug safety. At MD Biosciences toxicology studies are performed in rodents or pigs and are GLP and regulatory compliant. We assist with the study design and consider components like dosing route/regimen and dosing levels. Common readouts include clinical signs, body weight, clinical pathology, organ weights, gross pathology, histopathology and biomarkers. Samples are collected throughout the study for downstream analysis, and tissues are collected for necropsy and pathology. Additionally, we can streamline the regulatory process through our expertise in SEND data standardization and eCDT tables.
Sample Data
-
Rodent PK/PD Data
PK and tissue distribution in mice: comparison between IV and PO route of administration in mice.
GI data below. Collection feces can reflect the pharmacokinetics in the GI.



Pharmacokinetics in other organs.






-
Pig PK/PD Data
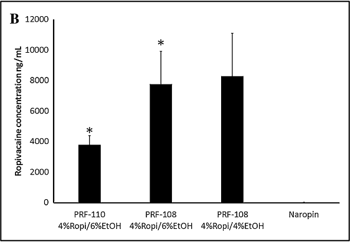
PK: systemic exposure of ropivicaine following local administration. Long exposure following PRF-108 treatment.

Ropivicaine concentration in wound fluid at 48 hours post local administration.
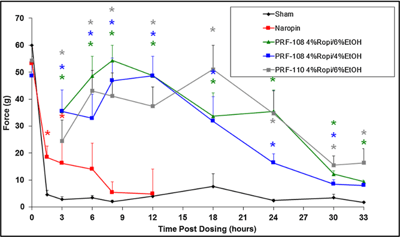
PD: long-acting activity of PRF-108 following local administration (ropivacaine formulation).
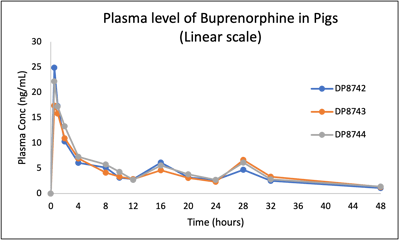
PK of buprenorphine in pigs following 0.2 mg/kg administration using IM route
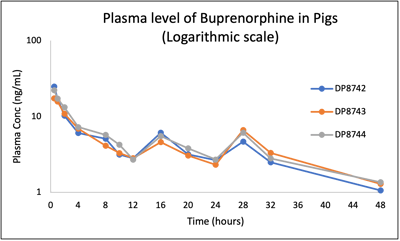

Review our publication:
Castel D, Naveh M, Aharon A, Doron O, Meilin S. Prolonged Analgesic Effect of PRF-108 and PRF-110 on Post-operative Pain in Pigs. Pain Ther. 2016;5(1):29-42. doi:10.1007/s40122-015-0043-9 -
Pig Toxicology Data
Exparel (106.4 per pig)
Bupivacaine effect on pain relief:

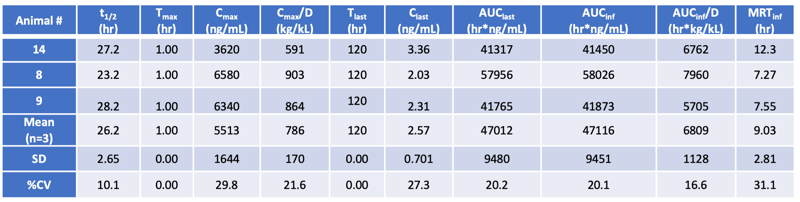
Bupivacaine plasma concentration:

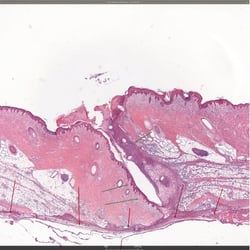
Toxicology of the injected site (magnification X 2):
Green arrows indicate the relatively thin and well-defined immature granulation tissue which extends through the dermis.
Red arrows indicate the relatively wider extension of the immature granulation tissues in the subcutis.
Bupivicaine PK data:
Clonidine testing
Review the publication: Wilsey JT, Block JH. Sustained analgesic effect of clonidine co-polymer depot in a porcine incisional pain model. J Pain Res. 2018;11:693-701. Published 2018 Apr 9. doi:10.2147/JPR.S157018
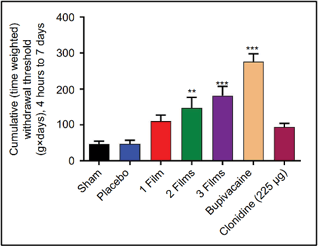

PK: plasma concentration following administration of a single clonidine film.
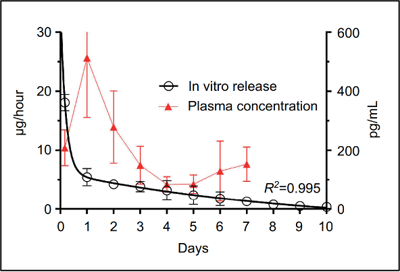
Plasma Cmax was 279±50.1 pg/mL at 24 hours, falling to 149±26 pg/mL at 72 hours.
READY TO DISCUSS YOUR PROGRAM?
At MD Biosciences, we’re your dedicated partner in drug development, offering tailored study designs, translational models, and comprehensive endpoint assessments. With short lead times, competitive pricing, and deep scientific expertise, we help move your research forward efficiently.